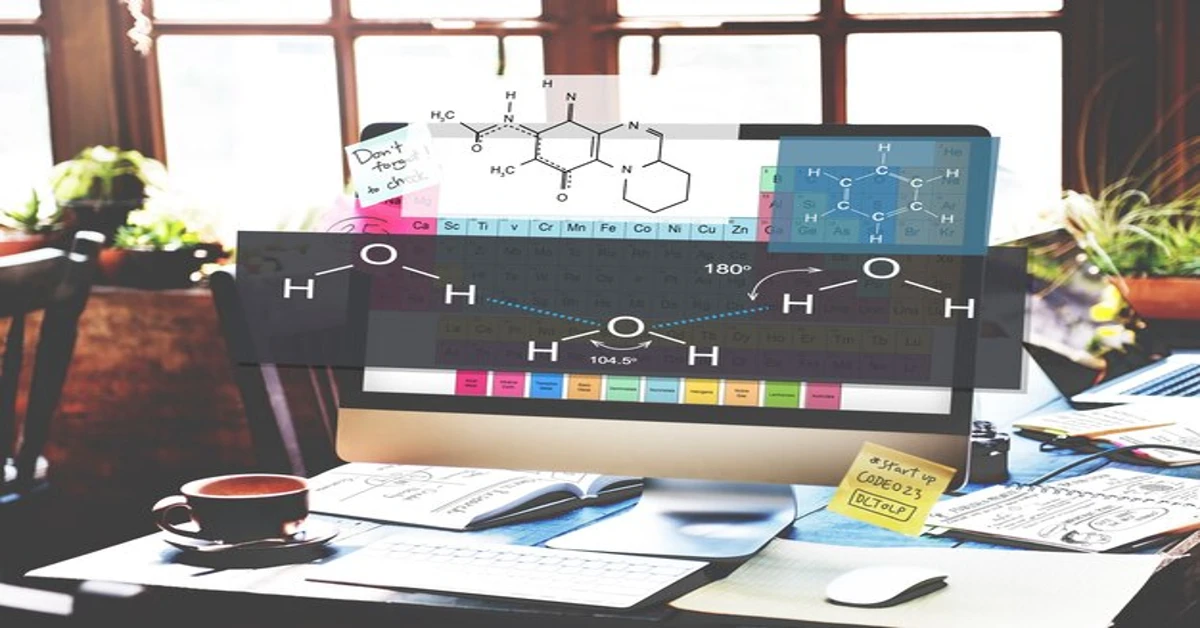
Understanding the building blocks of chemistry can open a world of possibilities. Among these essential components are HCOOCH, CH2, and H2O. These molecules play vital roles in various applications across multiple industries. From organic synthesis to the fragrance and flavoring sector, their significance cannot be overstated.
As we dive deeper into understanding HCOOCH CH2 H2O, we’ll explore how they interact within chemical processes and contribute to innovative solutions in research and development. Join us on this fascinating journey from molecular structures to real-world applications!
Understanding HCOOCH CH2 H2O
HCOOCH, commonly known as formate ester, is an organic compound that plays a crucial role in the chemistry of esters. Its structure comprises a formyl group attached to a methylene (CH2) unit, making it versatile for various applications.
CH2 represents the methylene group, which is vital in the formation and reaction of numerous chemical compounds. This simplicity allows it to participate in diverse reactions while contributing to molecular stability.
Water (H2O) acts as a universal solvent and often participates in hydrolysis reactions involving HCOOCH. The interplay between these three molecules opens up exciting avenues for exploration within organic chemistry and industrial usage.
The Significance of Organic Esters
Organic esters play a crucial role in various chemical processes. Their unique structure allows them to participate in reactions that form diverse compounds, making them essential in organic synthesis.
These compounds are widely recognized for their pleasant fragrances and flavors. They are often used in food products, perfumes, and cosmetics. This versatility makes organic esters valuable across multiple industries.
Additionally,HCOOCH CH2 H2O serve as solvents and intermediates in manufacturing. Their ability to dissolve other substances enhances production efficiency while maintaining desirable properties. As such, they contribute significantly to advancements in materials science and industrial applications.
Organic Synthesis
Organic synthesis is a fascinating branch of chemistry focused on constructing organic compounds through various chemical reactions. It plays a crucial role in producing complex molecules, which can be used to create everything from pharmaceuticals to agrochemicals. HCOOCH CH2 H2O serves as an essential component in many synthetic pathways.
The versatility of HCOOCH enhances the development of esters and other derivatives, making it invaluable for researchers and industrial chemists alike. By manipulating its properties, scientists can optimize reaction conditions for desired outcomes.
Moreover, understanding how water (H2O) interacts with these processes allows chemists to refine their techniques further. This synergy between HCOOCH CH2 and H2O fosters innovation within organic synthesis practices.
Fragrance and Flavoring Industry
The fragrance and flavoring industry thrives on innovation, with HCOOCH CH2 H2O playing a pivotal role. Organic esters like HCOOCH contribute to the creation of aromatic compounds that tantalize our senses. They are essential in crafting unique scents for perfumes and delightful flavors in food products.
HCOOCH is favored for its ability to blend seamlessly with other ingredients, enhancing overall profiles. Its presence can elevate a simple product into an unforgettable experience. This versatility makes it a sought-after ingredient among formulators.
Moreover, water (H2O) acts as a critical solvent in many formulations, ensuring even distribution of flavors and fragrances. The synergy between these components fosters creativity and offers endless possibilities within the industry.
Industrial Solvent
HCOOCH CH2 H2O are essential components in the field of industrial solvents. Their unique properties make them highly effective for various applications, from cleaning agents to paint thinners. This versatility allows industries to streamline their processes while maintaining efficiency.
In manufacturing, these compounds help dissolve materials or facilitate chemical reactions. Their ability to mix well with a variety of substances enhances productivity across numerous sectors, including pharmaceuticals and plastics.
Moreover, using HCOOCH as an industrial solvent often results in less environmental impact compared to traditional solvents. The balance between effectiveness and sustainability is crucial as industries seek greener alternatives without sacrificing performance.
Research and Development
Research and development play a pivotal role in exploring the full potential of HCOOCH, CH2, and H2O. Scientists are continually investigating their applications across various fields, from pharmaceuticals to materials science. This exploration helps identify innovative uses that can enhance efficiency and sustainability.
Through rigorous experimentation, researchers uncover new chemical pathways involving these compounds. These studies not only advance theoretical knowledge but also lead to practical solutions for industrial challenges. By understanding how these molecules interact under different conditions, breakthroughs become possible.
Moreover, collaboration between academia and industry fosters an environment ripe for innovation. Such partnerships ensure that research translates into real-world applications that benefit society as a whole while maintaining environmental integrity.
The Role of HCOOCH CH2 H2O in Chemistry
HCOOCH, also known as methyl formate, plays a crucial role in organic chemistry. Its unique structure allows it to participate in various chemical reactions. This versatility makes it an essential building block for synthesizing more complex compounds.
The presence of CH2 adds another layer of functionality to HCOOCH. This methylene group contributes to the molecule’s reactivity and stability during transformations. It often serves as a medium for introducing additional functional groups.
Furthermore, H2O acts as a solvent and reactant in many reactions involving HCOOCH. Water facilitates hydrolysis processes and helps create favorable conditions for achieving desired products. Together, these components enhance the scope of chemical investigations and applications across different sectors.
Applications of HCOOCH CH2 H2O in Industry
HCOOCH CH2 H2O finds numerous applications across various industrial sectors. This organic compound, commonly known as methyl formate, is particularly valued in the production of esters and solvents. Its low toxicity makes it suitable for use in formulations where safety is a concern.
Additionally, HCOOCH CH2 H2O serves as an excellent intermediate in chemical synthesis. Industries leverage its reactivity to create more complex molecules essential for pharmaceuticals and agrochemicals. The versatility of this compound allows for innovative processes that enhance efficiency.
Moreover, the fragrance and flavoring industry benefits significantly from methyl formate’s pleasant aroma profile. It acts as a key ingredient in creating enticing scents and flavors that appeal to consumers worldwide. Its role here highlights the importance of chemistry in everyday products.
Environmental Impact of HCOOCH CH2 H2O
The environmental impact of HCOOCH CH2 H2O is multifaceted. As a chemical compound, it can contribute to both pollution and sustainability efforts depending on its usage. When released into the environment, improper disposal or leaks can pose risks to aquatic life.
On the other hand, this compound plays a role in creating biodegradable products. Its properties are favorable for reducing reliance on more harmful chemicals in various applications. By promoting greener alternatives, it supports initiatives aimed at minimizing ecological footprints.
Additionally, understanding how HCOOCH CH2 H2O interacts with water systems is vital for assessing its long-term effects. Ongoing research informs regulations that help mitigate potential harm while leveraging benefits for industrial processes and sustainable practices.
Everything You Need To Know About HCOOCH CH2 H2O
HCOOCH, also known as formate ester, is a compound with significant importance in both organic chemistry and industrial applications. It consists of the formate ion (HCOO) bonded to CH2 groups. This composition makes it versatile for various chemical reactions.
Water (H2O) plays a crucial role in these reactions, acting as a solvent or medium that facilitates interactions between HCOOCH and other compounds. Its presence often influences the reaction pathways and outcomes.
Understanding HCOOCH CH2 H2O opens doors to numerous applications ranging from fragrances to solvents. The unique properties of this compound make it essential in research and development within the chemical industry, promising innovations across multiple sectors.
The Chemistry of Formate Esters
Formate esters, such as HCOOCH CH2, are fascinating compounds in organic chemistry. They result from the reaction between carboxylic acids and alcohols, forming an ester linkage. This process is essential for creating various functional materials.
The presence of the formate group offers unique properties like volatility and solubility. These features make formate esters ideal candidates for applications in fragrances and flavoring agents. Their pleasant aromas enhance countless consumer products.
Additionally, these compounds play a significant role in industrial processes as solvents or intermediates. Their ability to dissolve different substances allows them to facilitate reactions effectively, contributing significantly to advancements in chemical manufacturing and research endeavors.
Role of H2O in Reactions Involving HCOOCH CH2
Water, or H2O, plays a vital role in reactions involving HCOOCH CH2. It acts as both a solvent and a reactant, facilitating the transformation of organic compounds. In esterification processes, water is produced as a byproduct when acids react with alcohols to form esters like HCOOCH CH2.
Moreover, the presence of water can influence reaction rates and yields significantly. For instance, it can help stabilize intermediates during chemical reactions. This aspect is crucial for effective synthesis pathways in laboratories and industries alike.
Understanding how H2O interacts with HCOOCH CH2 enhances our knowledge of organic chemistry dynamics. The interplay between these molecules reveals opportunities for innovation across various sectors—be it material science or pharmaceuticals—demonstrating that every component has its unique contribution to chemistry’s vast landscape.